Background: As a therapy based on immune cells, CAR T cells treatment elicits an acute response involving both innate and adaptive immunity which is followed by a process of resolution. A major interest is to identify, within the tumor microenvironment (TME), factors that influence the activity and potency of anti-CD19 CAR T cells. The B-ALL TME provides factors that protect leukemia and, conversely, suppress effector T cells. However, studies on the interaction between the bone marrow (BM) TME and CAR T cells are still limited. We hypothesized that the immunological niche of the BM reacts to CAR T-cell-mediated inflammation by modulating the immune response through the activation of inhibitory pathways and molecules.
Methods: We conducted an academic phase I/II study (NCT03389035) with donor-derived anti-CD19 CAR T cells generated with Sleeping Beauty transposon (CARCIK-CD19) in B-ALL patients relapsed after alloHSCT. Hematology laboratory data, CAR T,and CD3+ cell kinetics were analyzed in 43 pediatric and adult B-ALL patients who underwent commercial and investigational anti-CD19 CAR T cell treatment. The scRNA-seq libraries of BM samples were generated using Chromium Single Cell 5′ Reagent Kit of the 10x Genomics (v3.1). Validation was performed in 20 pre-/post- CAR-T matched BM samples by a 30-color flow cytometry panel.
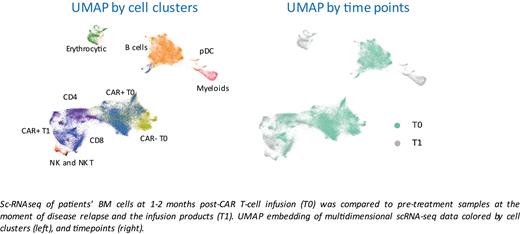
Results: Following the peak of expansion, CAR T-cell counts decreased in the third and four-week post-infusion. A similar dynamic of expansion and contraction was observed in CD3+ T cells, total white blood cells (WBC), and monocytes, indicating that a widespread resolution of CAR T-driven inflammation occurs during the first month after infusion. Interestingly, the intensity of the response seems to differ depending on the cell product used. To elucidate the mediators involved in the resolution of CAR T-cell-mediated inflammation, the single-cell transcriptome of patients' BM cells at early time points post-CAR T-cell infusion (1-2 months) was compared to pre-treatment samples at the moment of disease relapse and the infusion products. Eighteen sequencing libraries were analyzed, 6 libraries per patient, for a total of 71407 high-quality cells at an average of 65000 reads per cell. Unbiased clustering of BM cells and infusion products was performed by UMAP embedding. Notably, extensive integration of the dataset coming from the individual patients was observed.The more representative clusters were classified into infusion product, CD4 and CD8 endogenous population, B cells, myeloid cells, pDC, NK, and NK-T cells. We observed profound changes in the composition of BM after CAR T cells infusion compared to pre-treatment samples. Complete disappearance of the B cells associated cluster was observed after treatment. CAR T-cell treatment generated a remodeling of the BM microenvironment, with an increase in the myeloid cells (specifically monocytes), NK, NK-T, and exhausted CD8+ T cell populations. After CAR T-cell infusion, myeloid cells displayed a higher resemblance to myeloid-derived suppressor cells (MDSCs). GeneSet Enrichment Analysis (GSEA) showed significant enrichment in pathways associated with immunosuppression both in the myeloid compartment and in endogenous T cells and in CAR T cells. Of note, the genes involved are also strictly correlated to the generation of terminally exhausted T cells and the emergence of MDSCs. Spectral flow cytometry-based experiments validated our observation of an increase of monocytes, MDSC-like cells, NK, NK-T, and CD8 terminal cells. Modeling intercellular communication using NicheNet suggested the induction of immunosuppressive genes in myeloid cells by both CAR T cells and endogenous T cells. Using the same tool with all niches as the sender and T cells as the receiver, we observed that myeloid cells induced significant signaling in both CAR T cells and endogenous T cells.
Conclusions: Through sc-RNAseq and spectral flow cytometry, we have characterized changes in the BM TME after CAR T cell therapy. Specifically, CAR T cells-mediated myeloid activation is associated with pathways of immune dysregulation that may dampen CAR T cell expansion and antagonize the effects of the therapy. Furthermore, these data support the hypothesis that transcriptomic interrogation can infer pathways relevant to the communication between CAR T cells and the TME.
Disclosures
Biondi:Agmen: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Colmmune: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galapagos: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal